Today, the MACRAMÉ Project for the development of Advanced Characterisation Methodologies to assess and predict the Health and Environmental Risks of Advanced Materials, kicked off its three years’ research and innovation (R&I) activities at the first meeting of the MACRAMÉ Consortium in Brussels.
The Project is funded by the European Commission’s Horizon Europe Framework Programme (grant agreement ID: 101092686), as well as the Swiss State Secretariat for Education, Research & Innovation, and United Kingdom Research and Innovation, to develop methodologies for the detection, characterisation and quantification of Advanced Materials (AdMas) during their processing and product life cycle, including recycling/end-of-life, and to assess the resulting impact on health and the environment.
MACRAMÉ will prepare standardisation and harmonisation, as well as regulatory validation, the wide-spread applicability and application of the developed test and characterisation methods, and demonstrate their effectiveness and efficiency concerning existing, market-relevant industrial materials, processes and products.
Having formally started the started the Project on the 1st December 2022, the Project Team used the 2-day kick-off meeting to re-confirm its ambitious three-year R&I Strategy, while canvassing feedback and support from both the Project’s External Advisory Committee (i.e. an international groups of external experts from regulatory organisations, international, policy-informing organisations, large industries and research networks).
The R&I Strategy defines the life-cycle assessment for five market-relevant industrial MACRAMÉ Use-Cases. These define the selection of the MACRAMÉ R&I Activities and development of MACRAMÉ Methods, and the benchmarks chosen for monitoring the progress R&I. MACRAMÉ R&I Activities include a range of novel sample preparation techniques and ambitious quantitative detection and imaging methodologies that support reliable and reproducible determination of AdMas in different complex matrices (AdMa@CMs) and using inhalation as their main exposure route. By applying, combining and evaluating both established and novel inhalation toxicity tests a tiered approach to toxicity testing will be developed that will provide data on state-of-the-art characterised control materials for the MACRAMÉ Control Material Library. The library will serve future AdMa toxicological research. The ultimate MACRAMÉ Outcomes are proposals for harmonisation and (pre-)standardisation projects to be provided to and further elaborated with the relevant bodies, (i.e. OECD, VAMAS/CEN/ISO). The proposals will be founded on robust summary datasets, scientific documents and recommendations for hazard- and risk-assessment methodologies for AdMas in complex product matrices (AdMa@CMs). All data and information, obtained from external sources and generated during the Project, will be handled and stored in the MACRAMÉ Information Hub – the Project’s central information processor, whose interoperability is based on a Data Stewardship concept, designed according to IndustryCommons principles.
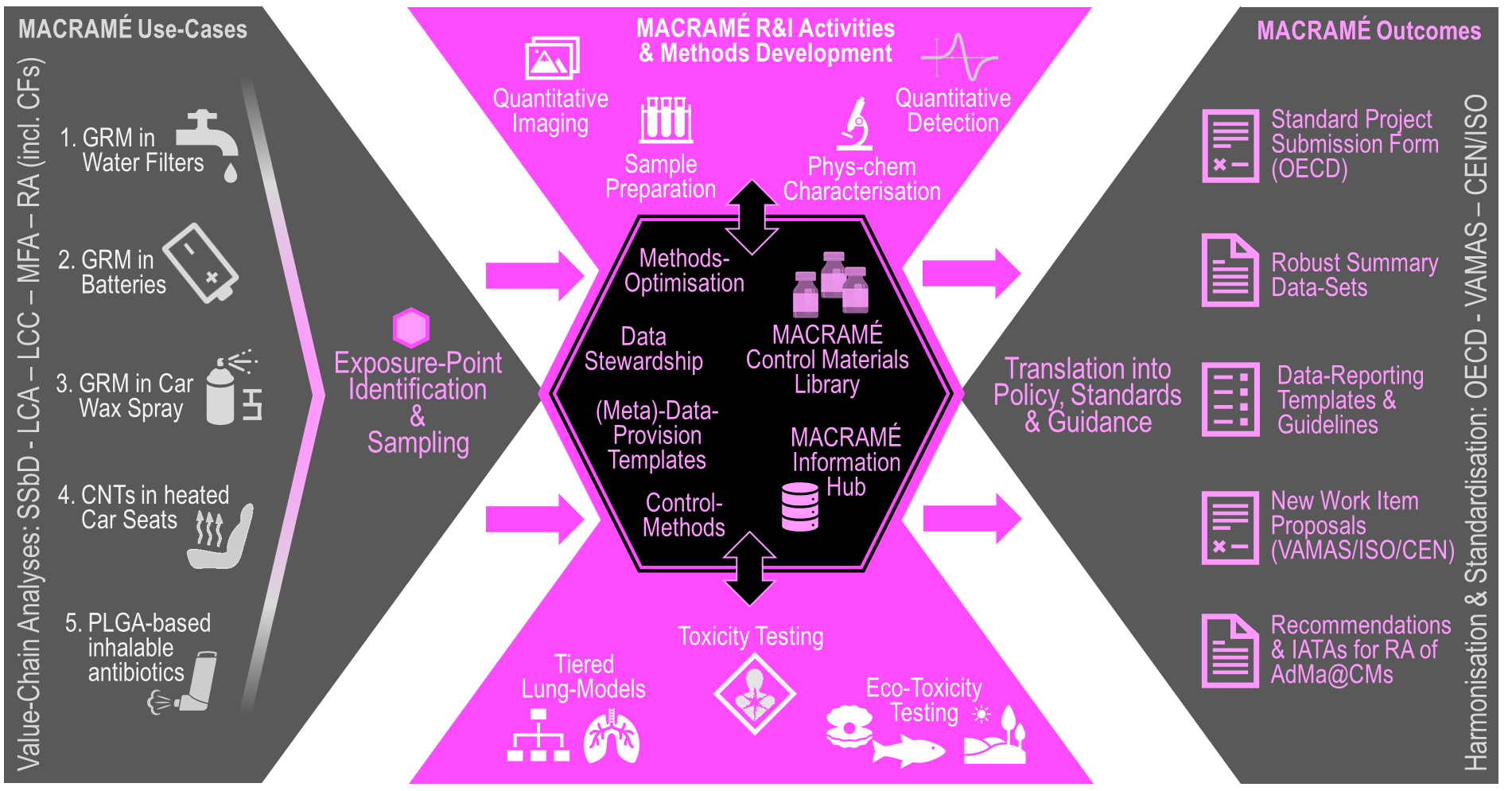
The resulting efficiency and effectiveness of MACRAMÉ Methods will be demonstrated through their application in Use-Case evaluations, using LCA-, LCC- and ‘Safe & Sustainable by Design’ (SSbD)-based (EWARN (2022)), highlighting benefits like reduced costs of regulatory compliance, by following a MACRAMÉ Safety & Sustainability Matrix. This matrix will be a modular building block for MACRAMÉ’s information-transferring interfaces for different scientific and regulatory communities, and thus provide a stepping-stone for Europe’s route towards a ‘one substance – one assessment’ approach (European Green Deal (2019)) and promote an open strategic autonomy (ETUI (2021)) through key enabling and emerging technologies, including digital ones.
- Specific Objective 1: Interfacing Communities and Streamlining Methodological Approaches to assess the Risk of AdMa@CMs
- Specific Objective 2: Assessment of market-relevant Exposure Points and representative Sampling along the Life-Cycle of five MACRAMÉ Use-Cases
- Specific Objective 3: Characterisation – Development & Harmonisation of Methodological Approaches for the Identification, Physical-Chemical Characterisation and Quantification of inhalable AdMa@CMs
- Specific Objective 4: Toxicity Tests – Development of new Advanced Methodologies & Harmonisation of existing Methodological Approaches for human and environmental Hazard Characterisation of inhalable AdMa@CMs
- Specific Objective 5: Hazard Assessment – Development of the scientific Background to foster regulatory Acceptability of Methods and Methodologies for the Physical-Chemical and Hazard Characterisation of AdMa@CMs
- Specific Objective 6: Establish improved Data-Reporting Guidelines and deliver harmonised, FAIR Data for the Characterisation of AdMas at different Life-Cycle Stages
Follow this link to find out more about the MACRAMÉ Project.